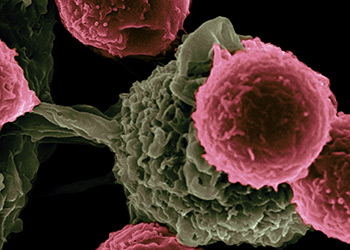
VICTOR SEGURA IBARRA AND RITA SERDA, PH.D., NCI, NIH
(www.statnews.com) – Novartis’ groundbreaking CAR-T cancer therapy is one big step closer to reaching patients.
A panel of outside experts convened by the FDA voted 10-0 Wednesday to recommend the approval of Novartis’ CAR-T therapy, called CTL019, for the treatment of children and young adults with advanced leukemia. The vote marks a pivotal milestone for this class of experimental treatment. The FDA is expected to make a final decision on approval by Oct. 3.
“I think this is most exciting thing I’ve seen in my lifetime,” said Dr. Tim Cripe, an oncologist with Nationwide Children’s Hospital on Columbus, Ohio and an expert who weighed in on CTL019 at Wednesday’s FDA panel.
CTL019 is a customized treatment made by harvesting patients’ white blood cells and rewiring them to home in on tumors. It’s the first CAR-T therapy to come before the FDA, leading a pack of novel treatments that promise to change the standard of care for certain aggressive blood cancers.
The FDA is also reviewing a CAR-T from Kite Pharma for the treatment of adults with advanced and aggressive lymphoma. An approval decision for the Kite CAR-T is expected on Nov. 29.
