
CureSearch Mission
Our mission is to end children’s cancer by driving targeted and innovative research with measurable results in an accelerated time frame.
CureSearch Vision
Advance better, less-toxic treatments to ensure all children with cancer live long, healthy lives.
Message from our CEO
At CureSearch, we are dedicated to accelerating the development of new, less-toxic childhood cancer treatments. Our goal is to save children, adolescents and young adults NOW and enable them to live long, healthy lives—free from the debilitating side effects that can accompany current, yet often decades old, treatments. It is our passion to drive measurable progress, and with your help, we are achieving real outcomes. As we close out a successful 2018 and dive into a promising 2019, we are honored to provide these mission-critical updates from the last six months. I'm particularly excited to share our most impactful highlights.
In November, we announced up to $7 million in new award funding across our three grant programs. These grants span all stages of the research continuum, each engineered to create new therapies that will reach kids as quickly as possible. Our expert scientific review committee is currently evaluating highly innovative applications from best-in-class researchers, and we look forward to announcing our new grant recipients this spring.
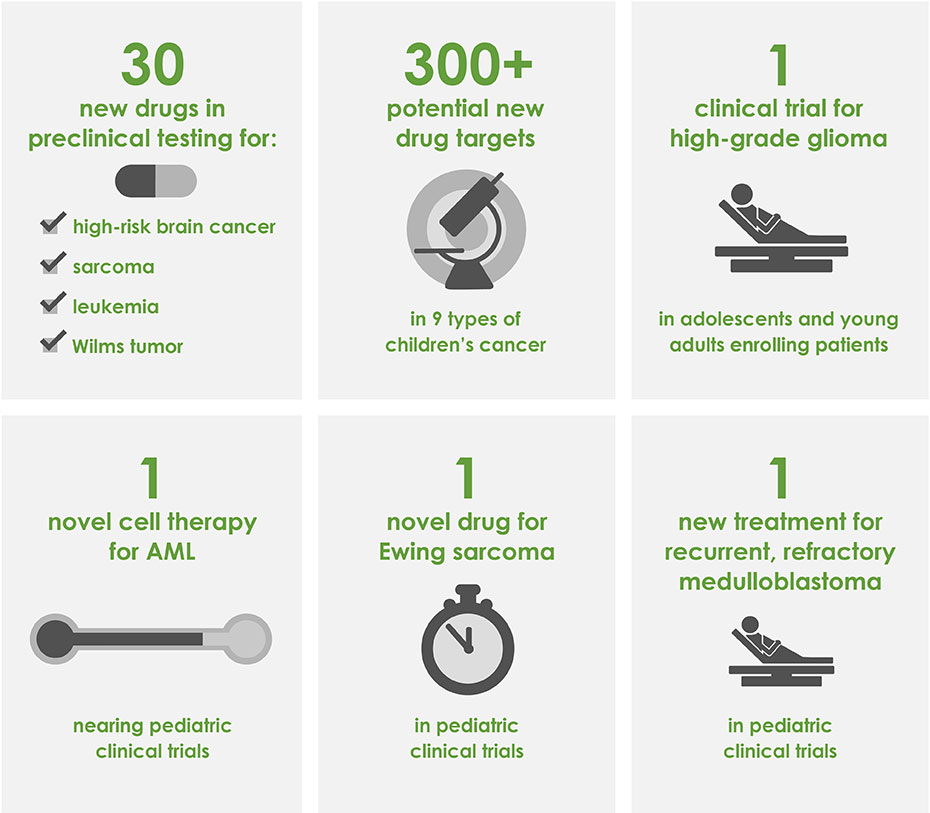
Our cumulative impact now includes 30 new drugs in preclinical testing and the identification of more than 300 new potential drug targets in 9 different types of children's cancer.
We're also proud to share that our Campaign Development team finished out a successful 2018, raising nearly $1.5 million through our fundraising campaigns Ultimate Hike, Superheroes Unite! (now CureSearch Walk), CureSearch Challenge and CureSearch Gold—all while keeping expenses under accepted industry standards. These unrestricted funds help to support our research, volunteer training, patient education and programs.
For over thirty years, CureSearch has been driving research to end children's cancer. Our accomplishments are possible only because of your commitment as passionate supporters, partners, event participants and volunteers. I sincerely thank you, for you are the cornerstone that helps us save children's lives.
Sincerely,

Kay Koehler
CEO, CureSearch for Children's Cancer
CureSearch Research Strategy Updates

Advancing the Development
of New, Less-Toxic Treatments
In November 2018, we announced new funding opportunities for all three of our grant programs:
- Our Young Investigators portfolio which provides seed funding for bright researchers, early in their careers, pursuing exciting, novel ideas.
- Our Acceleration Initiative program which addresses the largest obstacles in children's cancer research, driving translational research to the clinic.
- Our Catapult Awards which advance the strongest research into clinical trials and ultimately drug development.
This new funding will support projects that address areas of high unmet need in pediatric cancer research, including high-risk, relapsed or metastatic disease, adolescent and young adult patient populations, and/or the use of novel immunotherapy targets or modalities for treatment.
CureSearch grant recipients will be announced in the spring of 2019.
Inaugural Catapult Award Update
Project: Exploiting mutant IDH1/2-induced DNA repair defects in pediatric glioma |
"CureSearch funds all stages of research, from the young investigator to the established, and it truly bridges the gap to the clinic. CureSearch funding has been essential for my group to develop critical infrastructure in our laboratory, which we will use for years to come. They're involved in every step of the process—the CureSearch research strategy will benefit children by getting new therapies faster into the clinic. I cannot think of any other pediatric cancer foundation that operates at the level of CureSearch." - Dr. Ranjit Bindra |
Catapult Impact Update:
This inaugural Catapult Award will fund a phase 1 clinical trail testing a novel treatment for pediatric glioma, an aggressive brain cancer with a 5-year survival rate of less than 25%. If successful, Dr. Bindra's trial will lead to a far less toxic standard of treatment, reducing lifelong damaging side effects for patients and increasing the overall survival rate. The trial will open in 2019 and be conducted at 18 hospitals across the country.
Dr. Bindra was previously a very successful CureSearch Young Investigator, a testament to our commitment to funding the most promising research through all phases of the continuum.
Young Investigator Program Updates
 Loretta Li, MD
Loretta Li, MD
Dana-Farber Cancer Institute
Project: JAK2 inhibition and degradation in B-Cell Acute Lymphoblastic Leukemia
Dr. Li's Impact:
Dr. Li is developing a treatment for a high-risk subtype of ALL which occurs in 15% of ALL patients. Now six months into her project, Dr. Li has identified three new compounds that are able to stop the activity of JAK2, and she has begun to perform initial testing to determine how well they work and how well they are tolerated. In addition, Dr. Li is beginning to assess the activity of molecules that promote the destruction of the JAK2 protein, ultimately aiming to prevent the development of resistance mutations which are a current challenge when using inhibitors.
 Avery Posey, PhD
Avery Posey, PhD
University of Pennsylvania
Project: Polysialic acid-specific CAR T cells for the treatment of neuroblastoma
Dr. Posey's Impact:
Dr. Posey is working to develop a novel CAR T-cell therapy for neuroblastoma. CAR T-cell therapy has been successfully used for pediatric ALL patients and Dr. Posey is testing its effectiveness, using a disease-specific target, in neuroblastoma. Dr. Posey's goal is to advance this novel immunotherapy to clinical trials within 3-4 years. Now six months into his project, Dr. Posey is optimizing the molecular design of the chimeric antigen receptor (CAR) to develop the most robust interaction with the neuroblastoma-specific target, polySia. A strong interaction between the CAR T cells and polySia will ensure efficient targeting of neuroblastoma cells and optimal efficacy against the tumor. Dr. Posey's research group has developed two CAR Ts and tested them in five cell lines. They will continue to improve upon their design in the coming months.
Acceleration Initiative Program Updates
 Andrew Kung, MD, PhD
Andrew Kung, MD, PhD
Memorial Sloan Kettering Cancer Center
Project: Integrative analysis to identify new therapies for pediatric sarcoma
Dr. Kung's Impact:
Dr. Kung is advancing novel therapies for high-risk pedatric sarcomas and has developed an original method to identify the critical signaling nodes that drive malignancy. To date, Dr. Kung and his team have collected samples from over 150 sarcoma patients for analysis and creation of cell lines and animal models in which to test promising therapeutics. They have identified six potential targets that could be actionable in all sarcomas and are assessing inhibitors of these targets in their sarcoma models.
 Richard Gilbertson, PhD
Richard Gilbertson, PhD
Cambridge University
Project: Targeted therapies for high-risk pediatric brain tumor subtypes
Project is now complete.
Dr. Gilbertson's Impact:
Dr. Gilbertson began his project with the aim to provide clinical colleagues with route, dose, and schedule data of top candidate therapies for immediate translation into clinical trials. As a result of his preclinical data, St. Jude has opened a clinical trial for recurrent, refractory medulloblastoma patiens and a trial for choroid plexus carcinoma is forthcoming. In addition to the progress that Dr. Gilbertson has made towards promoting clinical trials, he has performed a high-throughtput drug screen, assessing over 1.2 million compounds for effectiveness against high-risk brain cancers and has developed 15 novel animal models using tissue from pediatric brain cancer patients.
 Maria-Grazia Roncarolo, MD
Maria-Grazia Roncarolo, MD
Stanford University
Project: Cell therapy methods to improve AML patient outcome post allo-HSCT
Project is now complete.
Dr. Roncarolo's Impact:
During her three years of CureSearch funding, Dr. Roncarolo developed a novel cell-based therapy for acute myeloid leukemia (AML) patients to improve upon standard treatments. While effective in many cases, standard therapy often results in a toxic side-effect called Graft versus Host Disease (GvHD) that can limit treatment success. GvHD occurs when immune cells donated to the patient for the purpose of killing the leukemia cells recognize the patient's tissue as 'foreign' and attack the patient's cells. The Roncarolo lab has developed a therapy that minimizes GvHD while maintaining the anti-leukemic effects of therapy. This novel therapy is anticipated to move into clinical trials within the next two years.
CureSearch’s Cumulative Impact
The dedication of volunteers, donors and partners continues to ensure that innovative research is advanced quickly through the research continuum. Ultimately, our impact is measured by the progress we make to advance new treatments to the kids who are depending on us.
Our Fundraising Campaigns
The impact of our fundraising campaigns was felt across the nation with events that raised funds for childhood cancer research and rallied communities around our shared mission. 2018 campaign fundraising highlights include:
 |  7 Ultimate Hikes + 3 Volunteer-led Hikes $757,031 |
 9 CureSearch Walks $447,717 |  |
 |  Over 40 DIY Events Across the Country $264,418 |
Thank you to all of our dedicated volunteers who
helped to make this possible.
Our Cancer Resources
Connect with CureSearch for resources, tools and information to help you navigate your cancer journey, including:
Board Members
CHAIRMAN OF THE BOARD SECRETARY TREASURER SAMUEL C. BLACKMAN, MD, PHD RICHARD J. O'REILLY, MD |
HANK ADAMS JARED D. BRANCAZIO, WMS CASON CARTER PAULA CARTER SHARI COLLIER ANNIE GOULD |
CARLEEN HAWN DAVID KUPIEC KATHY WANNER |
Our Generous 2018 Partners
Our work would not be possible without the extraordinary support of the following partners, including their generous employees and customers.
Catapult Partners
 |
 |
 |
Research Grant Partners
Mathile Family Foundation
Corn Endowment (Georgia Regents University)
Co-funding Partners
Parker Institute for Cancer Immunotherapy
Alan B. Slifka Foundation
Rising Tide Foundation for Clinical Cancer Research
Corporate Partners

 Ranjit Bindra, MD, PhD
Ranjit Bindra, MD, PhD