
CURESEARCH MISSION
Our mission is to end children's cancer by driving targeted and innovative research with measurable results in an accelerated time frame.
RESEARCH VISION
Improve children's cancer treatment and cures to deliver the potential for childhood cancer patients to lead long, healthy lives.
MESSAGE FROM THE CEO
 Our goal at CureSearch is to get better, less toxic treatments to kids in the clinic as quickly as possible. I'm happy to say that we're making real progress.
Our goal at CureSearch is to get better, less toxic treatments to kids in the clinic as quickly as possible. I'm happy to say that we're making real progress.
In the following report, you can read how our unique approach advanced a new treatment for Ewing Sarcoma to the cusp of filing for FDA approval for human clinical trials. To ensure that our research results in a path to the clinic, we've added another layer to our rigorous scientific review of project proposals. Our Industry Advisory Council reviewed the top selections for our Acceleration Initiative 3 grants to ensure that the project we’re funding has clinical potential, and further, that researchers have access to industry expertise during the course of their work.
However, funding great research and demanding results is not enough. Last February, we debuted the Catapult Initiative to propel discoveries over the Valley of Death and move treatments and cures out of the lab and into clinical trials and eventual commercialization. Our strategy allows for various thought leaders to convene and have access to the expertise, resources and opportunities needed to get new treatments to kids. Our second Catapult Summit will take place in San Francisco in February.
These are just a few of the initiatives that took place this year. Yes, they will help improve the odds of survival of the 40,000 children currently undergoing cancer treatment, but there is still much work to be done. When you donate to CureSearch, you accelerate research that transcends a broken research system and leads to cures. You make a difference for every boy and girl diagnosed with cancer.
Until there are cures,
Laura Thrall
CEO
CureSearch for Children's Cancer
CURES WITH INTEGRITY
Ending children's cancer is imperative. At CureSearch, our values guide us in how we are pushing forward to find cures and less-toxic treatments for children with cancer.
HONESTY & INTEGRITY
TRANSPARENCY & ACCOUNTABILITY
CREATIVITY & COLLABORATION
THE CURESEARCH DIFFERENCE
| We focus only on children's cancer. | |
| We're not here just to fund research, we're here to drive patient impact. | |
| Our Scientific Advisory Council, Industry Advisory Council, and Scientific Review Committee brain trust is unmatched. | |
| We invest in large-scale grants over a three-year timeframe, which allows our researchers to focus on their work, not fundraising. | |
| We hold ourselves - and our grant recipients - accountable through a novel measurement framework and frequent reporting to our constituents. | |
| We build strong, long-term partnerships with our funded researchers so that successful work is supported beyond the scope of their original CureSearch grant. | |
| We are bridging the 'valley of death' by creating unique partnerships with biotech and pharma to ensure that promising research makes it out of the lab and into the clinic. | |
| We propel discoveries over the Valley of Death and move treatments and cures out of the lab and into clinical trials by bringing together leaders in academia, industry, policy regulation, private foundations, as well as parents to accelerate the fight to end children's cancer. |
CURESEARCH COLLABORATION
By facilitating relationships between academic researchers and the pharmaceutical industry, we can get new cures and treatments out of the lab and to the children.
To facilitate academic-industry partnerships, in 2016 CureSearch formed The Industry Advisory Council (IAC), a team of experts from pharmaceutical companies supporting our efforts to drive drug development.
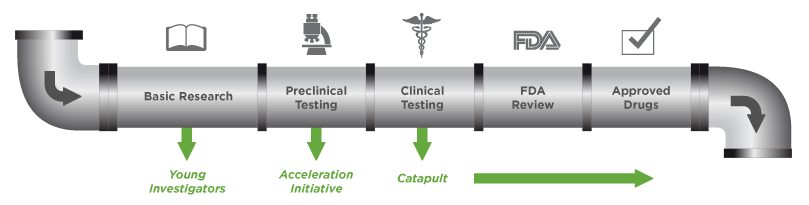
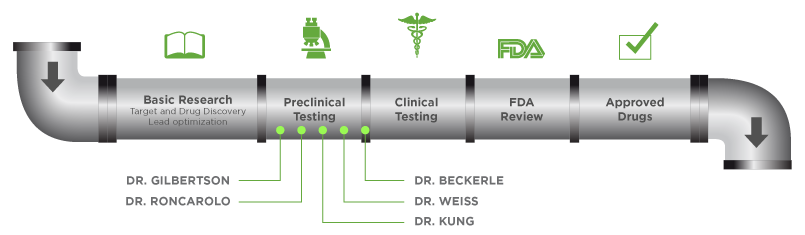
THE CURESEARCH RESEARCH PIPELINE
We recognize the fact that innovation has stalled in the search for cures. In response, we have taken a more entrepreneurial approach to funding children's cancer research.
By soliciting and funding the very best research at a large scale, for the long term, we are building a pipeline of promising treatments, from basic research all the way to clinical trials, that will dramatically accelerate our ability to end children's cancer.
PROPELLING DISCOVERIES
WITH CATAPULT
In the complex world of children's cancer research, no independent organization currently coordinates efforts between academic scientists, drug developers, and policy regulators to advance new treatments and cures. Through Catapult, CureSearch is proud to play the role of convener and collaborator to bring these key parties together in a way that is singularly focused on accelerating the commercialization of children's cancer treatments to save children now.
The ground-breaking Catapult initiative will:
- Propel promising treatments from laboratories to children by building on our unique expertise and connections within the childhood cancer space.
- Increase childhood cancer survival rates and reduce the cumulative burden of disease for survivors by providing research assets and expertise to overcome these barriers.
- De-risk the high cost of treatment development by funding clinical trials and related studies.
The 2nd Annual Catapult Summit will take place on February 26 and 27, 2017. CureSearch is excited to work with stakeholders to move Catapult to the next level and drive more treatments to the clinic and into commercialization.
 |
 |
ACCELERATION INITIATIVE
Through this $10M trailblazing initiative, CureSearch invests in the most innovative pediatric cancer research. We convene the foremost experts in children's cancer treatment and research to identify the greatest challenges facing the children's cancer field today. We then target grants that address the challenges in an accelerated timeframe.
All Acceleration Initiative research projects have the following characteristics:
| Highly innovative with the potential to break new ground in the field | Probability of clinical application in an accelerated timeframe | Ability to overcome scientific and therapeutic roadblocks to speed up the delivery of new and improved therapies |
ACCELERATION INITIATIVE DRUG
DISCOVERY AND DEVELOPMENT PIPELINE
ACCELERATION INITIATIVE 1
$5M over three years
(2013-2016)

Mary Beckerle, PhD
Huntsman Cancer Institute
University of Utah
Epigenetics
$1.73M 
"Without the financial support of this CureSearch award, we may have remained stuck at the stage in which we could identify and discuss the potential of a collaborative team with the goal of developing a new therapy for Ewing sarcoma. Thanks to the CureSearch award, a new collaboration was born with the goal of accelerating progress in the development of a new therapy for Ewing sarcoma."
- Mary Beckerle, PhD
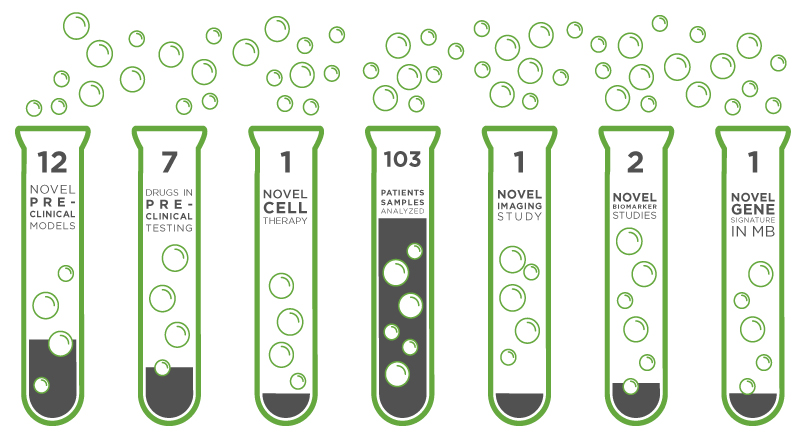
1 | Lead compound close to pediatric clinical trials |
1 | Novel biomarker study developed |
1 | Novel imaging study developed |
35 | Pediatric Ewing sarcoma biopsies |

William Weiss, MD, PhD
The Regents of the University of California, San Francisco
Epigenetics
$1.88M 
"Support from CureSearch has enabled us to identify unrealized vulnerabilities in MB that may nevertheless be targeted using existing or emerging drugs, being developed against epigenetic targets."
- William Weiss, MD, PhD
3 | Drugs for high risk MB in preclinical testing |
28 | Pediatric brain biopsies studied |
2 | Novel tumor model designs |
1 | Novel gene signature linked to high-risk MB |

Kathleen Sakamoto
MD, PhD
Stanford University
Immunotherapy
$1.37M*
*CureSearch is committed to research that has the highest probability of saving children as fast as possible. While we recognize the great promise in Dr. Kathleen Sakamoto's Immunotherapy project, due to a significant delay in the launch of the pediatric clinical trials, CureSearch discontinued funding in April of 2016.
We look forward to seeing Dr. Sakamoto's research progress, and encourage her to reapply.
ACCELERATION INITIATIVE 2
$1.69M over three years
(2015-2018)
Two awardees selected in partnership with
Rising Tide Foundation for Clinical Cancer Research.
 |
 |

Richard Gilbertson
MD, PhD
University of Cambridge
Targeted Therapies for High-risk
Brain Tumors
$835K 
"We are honored to work with Rising Tide and CureSearch on this exciting project...to improve the lives of children with brain tumors."
- Richard Gilbertson, MD, PhD
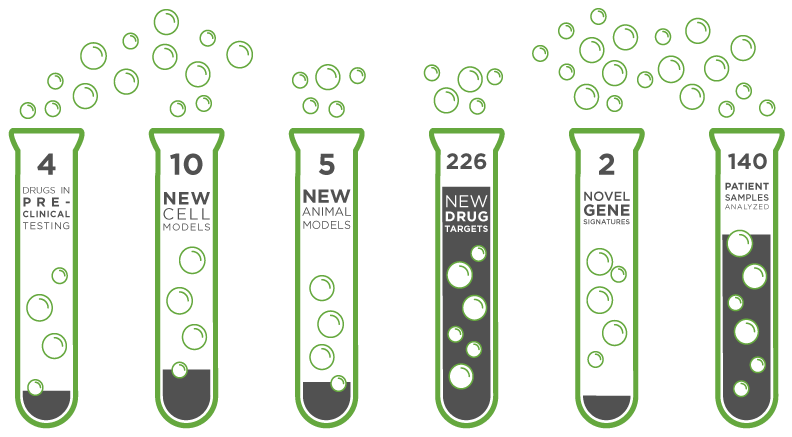
3 | High-risk brain tumor subtypes |
5 | New pre-clinical models developed |
3 | Compounds in pre-clinical testing |
1 | Novel pre-clinical trial design |

Maria-Grazia Roncarolo
MD
Stanford University
Cell-based Therapy for AML
$855K
"The funding provided to my lab by CureSearch and Rising Tide has enabled me to pursue research into cell therapy technology for AML with the aim of moving into clinical trials. Ultimately, this grant will help advance into the clinic a safer, more controllable cell-based immunotherapy."
- Maria-Grazia Roncarolo, MD
7 | New cell lines developed for novel cell therapy |
40 | Pediatric AML samples in pre-clinical testing |
1 | Clinical trial planned within three years |
ACCELERATION INITIATIVE 3
(2016-2019)
In May 2016, CureSearch announced the opening of the application window for the International Grand Challenge Awards, which address three critical challenges in pediatric cancer treatments:
| GRAND CHALLENGE 1: Use personalized medicine (molecular profiling of tumors) to improve diagnosis, treatment and outcomes for high-risk pediatric cancer patients. |
GRAND CHALLENGE 2: Develop targeted combination therapies that will reduce relapse and improve outcomes in high-risk pediatric cancers. |
GRAND CHALLENGE 3: Apply innovative technologies used in adult cancers to find new treatments for pediatric cancer. |
In November 2016, a total of $1.26M (USD) was awarded to Andrew Kung, MD, PhD, from Memorial Sloan Kettering Cancer Center in New York, New York. His project will study high-risk pediatric sarcomas - rhabdomyosarcoma, Ewing sarcoma, osteosarcoma - and identify key "master regulators" using a novel molecular profiling platform.
"Thank you for this fantastic news. We are delighted and look forward to a strong partnership to push this forward."

Andrew Kung, MD
Memorial Sloan Kettering Cancer Center
Integrative analysis to identify new therapies for pediatric sarcoma
$1.26M
1 | Novel approach to find genetic drivers of sarcoma |
1 | New animal model to be generated to test novel treatments |
3 | New drugs to be matched to genetic drivers for 3 pediatric sarcomas: rhabdomyosarcoma, osteosarcoma, Ewing sarcoma |
ACCELERATION INITIATIVE CUMULATIVE IMPACTS
1 Pediatric Clinical Trial for Ewing Sarcoma in 2017
ACCELERATION INITIATIVE
SUCCESS STORY

When the CureSearch Acceleration Initiative (AI) was launched over three years ago, its goal was to encourage researchers to focus on getting new treatments into pediatric clinical trials and eventual commercialization on the front end as soon as possible.
We encouraged researchers to focus on patient impact of their work, not just on publishing their results in academic journals. Now someone has done it.
Thanks to Nancy and Ralph Currey's support from the Nick Currey Fund, other generous donations, and a push for patient outcomes on the front end, Dr. Beckerle’s innovative AI project is advancing. She and her associates have developed a novel therapeutic drug that can stop the growth and spread of Ewing sarcoma. They are in the final stages of testing it, and expect to submit an Investigational New Drug (IND) application for FDA approval in January 2017.
Thank you to everyone who has joined us in our fight to accelerate the search for cancer cures. It's a fight we will continue until every child is guaranteed a cure.
 We fixed our goal on saving the Nicks of the future. We were so impressed with the caliber of physicians that the organization could draw upon in assessing research projects. We knew we could rely on their judgment to pick the best possible study to help us achieve our goal."
We fixed our goal on saving the Nicks of the future. We were so impressed with the caliber of physicians that the organization could draw upon in assessing research projects. We knew we could rely on their judgment to pick the best possible study to help us achieve our goal."
– Ralph and Nancy Currey
 "The strong direction of CureSearch leadership reminded us that the most important thing was progress toward the clinic, not academic publications. CureSearch provided exactly the kind of support and encouragement that enabled us to shift to team science, with clarity of purpose and the goal of advancing our science to the clinic."
"The strong direction of CureSearch leadership reminded us that the most important thing was progress toward the clinic, not academic publications. CureSearch provided exactly the kind of support and encouragement that enabled us to shift to team science, with clarity of purpose and the goal of advancing our science to the clinic."
- Dr. Mary Beckerle
 "By encouraging researchers to focus on getting treatments into the clinic where kids' lives can be saved, by connecting the expertise of our advisory councils to these projects, and by sticking to our guns, we're changing how research is done. That's a big paradigm shift, and a really important one."
"By encouraging researchers to focus on getting treatments into the clinic where kids' lives can be saved, by connecting the expertise of our advisory councils to these projects, and by sticking to our guns, we're changing how research is done. That's a big paradigm shift, and a really important one."
-Laura Thrall
YOUNG INVESTIGATORS
The CureSearch Young Investigator (YI) program is designed to attract early stage investigators and feed the pipeline for future Acceleration Initiative investigators. YI Grants fund transformational science designed to deliver the next generation of cancer treatment.
The first Young Investigator grant awarded $1.2M over two years and resulted in two clinical trials, 11 potential new therapies, and 360 new drug targets.
YOUNG INVESTIGATOR 2
$1.35M over three years
(2015-2017)
CureSearch awarded six new grants in 2015. After one year, each of the grantees has made substantial progress towards finding new treatments.
SIX YOUNG INVESTIGATOR 2 GRANTEES
|
|
SPOTLIGHT ON:
"We are tirelessly working towards better treatments for sarcoma, and it is only a matter of time before major advances will be seen. We depend heavily on the support of foundations like CureSearch, and thank those who support this research." |
YOUNG INVESTIGATOR 2 CUMULATIVE IMPACTS
87 Patients Enrolled in
1 Clinical Trial
MEET THE RESEARCHER:
DR. KARA DAVIS

"I am proud of the work we have done with the support of CureSearch in building the research and analysis pipeline to use single-cell studies for childhood leukemia with a true translational endpoint. This work has been a labor of love, taking seven years to get to this point with lots of ups and downs. We are certainly not at the end, really only the beginning, but getting the foundation in place and first manuscript submitted are still big milestones towards bringing our single-cell approach to clinical utility."
- Dr. Davis
Read our interview with Dr. Davis.
 SOLE BENEFACTOR |
COMMUNITY IMPACT AWARDS
In recognition of the importance of pyscho-social and caregiver resources and clinical trials in addressing the immediate needs of children with cancer, CureSearch launched the Community Impact Awards (CCIA) in 2015.
CCIA 2015 CUMULATIVE IMPACTS
| 120 | Patients received psychosocial care/services. |
| 380 | Children enrolled in clinical trials. |
| 130 | New treatments tested. |
CCIA 2016
CHILDREN'S CANCER RESOURCES
AT YOUR FINGERTIPS
|
The CureSearch CancerCare mobile app allows parents and caregivers to:
|
 |


BRINGING PEOPLE TOGETHER TO SUPPORT CHILDREN'S CANCER RESEARCH
CURESEARCH PARTNERS
Our work would not be possible without the extraordinary support of the following partners, including their generous employees and customers:

DONOR FUNDS AND ULTIMATE GIFTS
We are grateful to the families who have established a Champion or Legacy Fund to honor their child:
|
Jacob Koenigs 'Jakefest' Memorial Fund and Kiewit |
Luke McGuire Legacy Fund |
We are honored that these people have become members of the Legacy Society by including CureSearch in their will or trust:
The Howard and Janet Emery Living Trust
Jean B. Michael
Robert T. Ridley
EVENTS
New in 2017, CureSearch Superheroes Unite! will replace the CureSearch Walk. These events will still have the same focus of bringing together families, honoring children who have fought and are fighting cancer, and even a walk. But now it will be our top priority to make young cancer fighters feel like superheroes.

 Abby Rosenberg, MD, MS
Abby Rosenberg, MD, MS Kara Davis, DO
Kara Davis, DO Anthony Graves, MD, PhD
Anthony Graves, MD, PhD Andrew Lee Hong, MD
Andrew Lee Hong, MD Birgit Knoechel, MD, PhD
Birgit Knoechel, MD, PhD Ranjit Bindra, MD, PhD
Ranjit Bindra, MD, PhD