By Caitlyn Barrett, PhD – CureSearch National Director of Research & Programs
At CureSearch, we’re focused on driving the most innovative new therapies towards clinical trials where they’ll directly impact children in treatment today. Simply put, if a project can’t reach the clinic in an accelerated timeframe, we don’t fund it.
Our strategy is unique, and we’re delivering results.
On average, only 8% of cancer preclinical projects make it out of the lab and into clinical trials [1]. By comparison, 40% of CureSearch preclinical Acceleration Initiative projects advance to clinical trials where children are waiting for safer, more effective treatments. We’re funding the right projects and delivering new treatment options to patients in clinical trials right now.
This focus on translational research – bench to bedside – is critical. Limited federal funding generally supports basic or discovery phase research and, on the opposite end of the development timeline, late-phase clinical trials are supported by biotech and pharmaceuticals. There is a significant gap in funding for those areas in between, where translational, preclinical, and early-phase clinical trials lie.
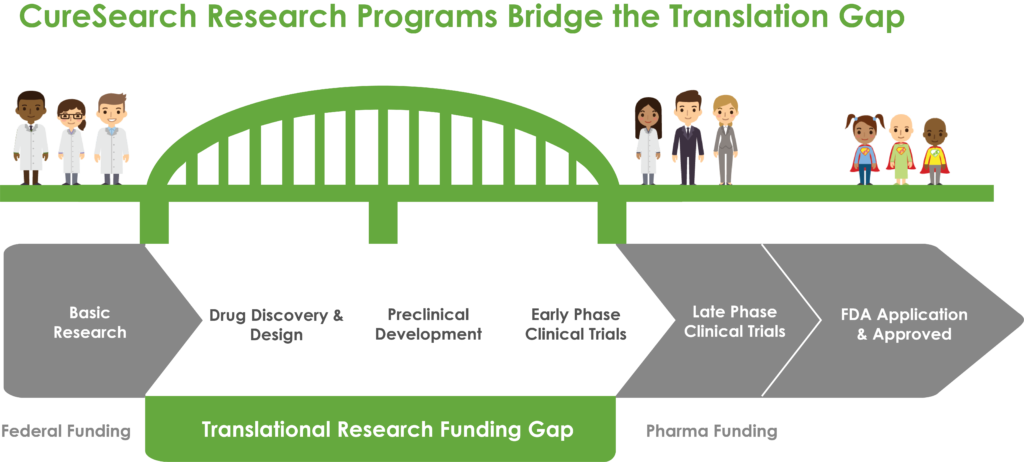
CureSearch’s funding efforts aim to fill that gap and ensure that promising new therapies make it into clinical trials. With over 60% of pediatric cancer patients participating in a clinical trial, it’s critical that innovate treatment options are available to them.
“While funding exists for new discoveries, little to no funding infrastructure is in place for catalysis and development of these discoveries into first-in-human clinical trials. I can think of no other foundation primarily focused on funding the developmental work necessary to bring forward new discoveries into groundbreaking clinical trials.” – CureSearch-funded researcher Dr. Elias Sayour
We’re not only demonstrating an incredible success rate in driving projects into clinical trials, but we’re also doing it at a faster pace. We see therapies identified in the lab move into clinical trials in 3.4 years compared to an average of 5.5 years [2]. This speed is essential as we remember that pediatric patients are often receiving decades-old treatments.
These impactful results are only possible with the ongoing support of our generous donors, volunteers, fundraisers, and partners. Together, we truly are making an impact for the kids who are counting on us.
| [1] | I. W. Mak, N. Evanview and M. Ghert, “Lost in translation: animal models and clinical trials in cancer treatment,” American Journal of Translational Research, vol. 6, no. 2, pp. 114-118, 2014. |
| [2] | H. Matthews, J. Hanison and N. Nirmalan, ““Omics”-Informed Drug and Biomarker Discovery: Opportunities, Challenges and Future Perspectives,” Proteomes, vol. 4, no. 3, p. 28, 2016. |
