CureSearch Research Programs
Our unique funding strategy focuses on addressing areas of unmet need in childhood cancer.
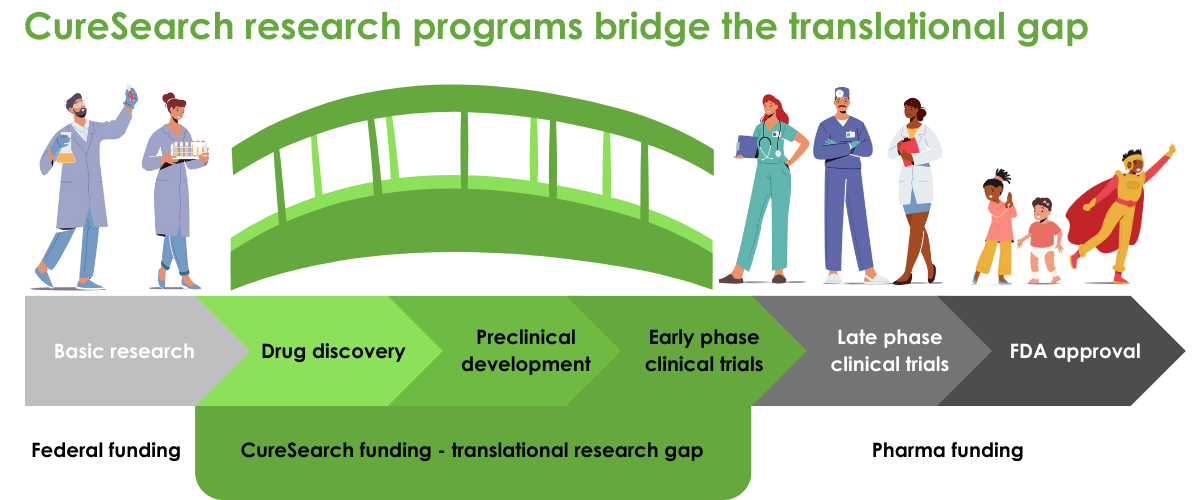
Our three distinct research programs fund both pre-clinical and clinical research, and are each designed to accelerate the pace of pediatric drug development and deliver new treatments directly to the children who so desperately need them.
- CureSearch Young Investigator Awards: Through our Young Investigator awards, we combat the loss of promising scientists from the field by providing significant financial support to investigators early in their research careers. These grants are limited to truly transformational science designed to deliver the next generation of cancer treatment to the clinic.
- CureSearch Acceleration Initiative Awards: Our Acceleration Initiative projects are highly innovative, address a significant challenge in pediatric cancer drug development, and have a strong probability of clinical application — ready to reach patients within three years.
- Catapult Awards Awards: Our Catapult Awards propel high-potential research out of the lab into the clinic and ultimately, to the kids who need it most. Our Catapult Awards provide funding for Phase 1 or Phase 2 clinical trials that advance promising therapies for pediatric cancer. The Catapult Award uniquely funds cutting-edge clinical trials that begin enrolling patients in less than a year, and every applicant must provide a development plan that demonstrates their ability to move into later-phase clinical trials if successful.
We’re laser-focused on accelerating the development of new childhood cancer treatments.
We only fund projects with commercial potential, anticipated to reach patients in the clinic or marketplace within three to five years. Our translational, preclinical, and clinical-stage awards give preference to areas of high unmet need – the cancers with the lowest survival rates, fewest or most damaging treatment options, and populations that are underserved, including adolescents and young adults. Within the context of unmet need, we prioritize innovative therapies that have strong potential to lead to more effective and less-toxic therapies, including novel targeted therapeutics, immunotherapy, and combination therapies. We support international research because the next game-changing discovery can come from anywhere, and international collaborations can unite the greatest minds for the development of the most effective cures.
We’re changing the status quo:
- Our research is focused on saving kids. We identify and fund high-potential research projects with an aggressive timeline to meet our end goal: developing treatments to save kids now.
- CureSearch requires milestones, measures progress, and demands accountability from our researchers to deliver patient-centered outcomes.
- CureSearch is armed with the best-in-class councils advising with scientific and industry expertise to ensure research is engineered to advance to the marketplace.
We won’t stop until more and better children’s cancer treatments are out of the lab and into the clinic and commercialization, ensuring more children live long, healthy and productive lives.
